XuQian'sGroup,School of Public Health, NanjingMedical University /HongqianGuoandXiaozhi Zhao's Group,Nanjing Drum Tower Hospital,theAffiliated Hospital of Nanjing University Medical School
Newmechanismunderlying inhibitionof PTEN activity by the oncometabolitefumarate
Fumarate isan intermediate metabolite of the tricarboxylic acid cycle. Catalyzedby fumarate hydratase (FH) into malate, fumarate does not accumulateexcessively in normal cells. Studies have shown that, due tometabolic reprogramming, fumarate accumulates in a variety of tumorsand promotes tumor progression. Therefore, fumarate is considered asan oncometabolite. However, the mechanism underlying fumarate-exertedtumorigenesis remains unclear.
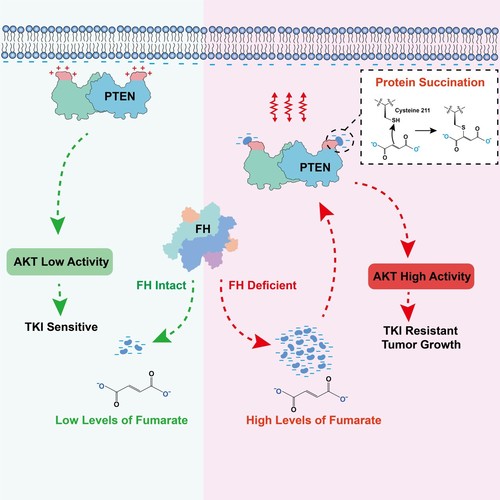
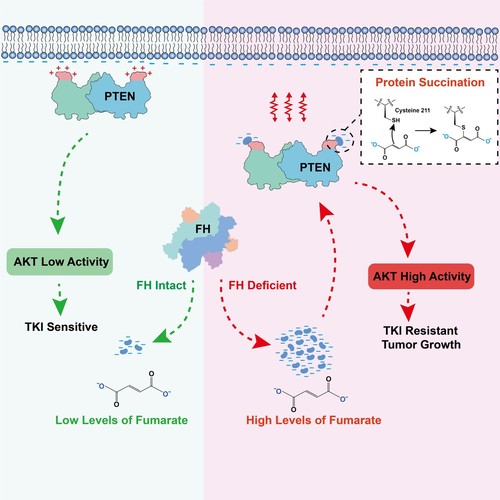
FH is theonly catabolic enzyme for fumarate in the cell. The accumulation offumarate caused by inactivate mutations of FH gene serves a molecularpathological feature of human type 2 papillary renal cell carcinoma(PRCC2). The current study demonstrated that the inactivate mutationrate of FH was 47% (48/102) in PRCC2 clinical samples. To furtherinvestigate the oncogenic mechanism of fumarate, the researchers dida screen assay and found that both FH inactivate mutations andfumarate accumulation significantly activated the PI3K/AKT signalingpathway. Subsequently, they found that this was mainly due to theinhibition of PTEN activity by fumarate. Through mass spectrometryanalysis, the researchers found that cysteine 211 residue (C211) ofPTEN is modified by fumarate through the Michael addition reaction, apost-translational modification termed protein succination. C211 islocated adjacent to CBR3 domain that mediates the binding of PTEN tothe cellular membrane. PTEN C211 succination increases the localnegative charges in the region of CBR3 domain, resulting in decreasedmembrane binding of PTEN and diminished inhibitory effect on thePI3K/AKT pathway, which subsequently promotes tumor growth andtherapeutic resistance of PRCC2. PTEN C211S mutant or overexpressingwild-type FH effectively restores tumor suppressor activity of PTENand inhibits cell proliferation of tumors. The combination of AKTinhibitors improved the sensitivity of PRCC2 to receptor tyrosinekinase inhibitors (TKIs)-targeted therapy in clinic. Finally, theresearchers also found that levels of PTEN C211 succination arepositively correlated with AKT activation and that both high levelsof PTEN C211 succination and AKT activation predict poor prognosis inPRCC2 patients.
Xin Ge, apostdoctoral fellow, and Mengdie Li, a doctoral student of , at theSchool of Public Health, Nanjing Medical University, are the co-firstauthors of the paper. Professor Xu Qian at the School of PublicHealth, Nanjing Medical University, and Professor Hongqian Guo andAssociate Professor Xiaozhi Zhao at the Department of Urology ofNanjing Drum Tower Hospital, the Affiliated Hospital of NanjingUniversity Medical School, are the co-corresponding authors of thepaper.
Figure.Succination modification of PTEN by fumarate, abrogates the bindingof PTEN with the cellular membrane, result in activation of PI3K/AKTsignaling for promoting tumor malignant progression and therapeuticresistance
PublicationDate:April2022
Journal:MolecularCell
AcriticalTitle:Fumarateinhibits PTEN to promote tumorigenesis and therapeutic resistance oftype 2 papillary renal cell carcinoma
Linkto the article:https://pubmed.ncbi.nlm.nih.gov/35216667/