Dr. Qian and his group reveals a critical bifunctional role of TMZ in GBM treatment.
Prof. Xu Qian's group, in collaboration with Prof. Yongping You from the Department of Neurosurgery at the First Affiliated Hospital of Nanjing Medical University and Prof. Zhimin Lu from the Institute of Translational Medicine at Zhejiang University, have recently published an article titled "Hypoxanthine phosphoribosyl transferase 1 mediates temozolomide metabolism to activate AMPK signaling pathway driving chemoresistance in glioblastomas" in Nature Communications. This study demonstrates that hypoxanthine phosphoribosyl transferase 1 (HPRT1) efficiently catalyzes the conversion of Temozolomide (TMZ) metabolite AICA to 5-aminoimidazole-4-carboxylated ribosome-5-phosphate (AICAR), subsequently activating the AMPK signaling pathway and promoting chemotherapy resistance in glioblastoma (GBM).
TMZ has been utilized for the treatment of GBM for over three decades; however, it has only resulted in a moderate extension of patient survival time. Gaining an understanding of the underlying mechanism responsible for intrinsic chemoresistance to TMZ is crucial in order to enhance treatment outcomes. In this study, Xu Qian's laboratory revealed a pivotal dual role played by TMZ in GBM therapy. The methyldiazonium cations derived from TMZ deliver methyl groups to purine bases within DNA, inducing DNA damage. Conversely, AICA derived from TMZ counteracts the DNA damage caused by methylated purines through promoting DNA damage repair.
This study demonstrates that 5-aminoimidazole-4-carboxamide (AICA), derived from TMZ, undergoes conversion to AICA ribosyl-5-phosphate (AICAR) within GBM cells. This enzymatic conversion is facilitated by hypoxanthine phosphoribosyl transferase 1 (HPRT1), which exhibits high expression levels in human GBMs. As the authentic activator of AMP-activated protein kinase (AMPK), TMZ-derived AICAR stimulates AMPK activation and subsequent phosphorylation of threonine 52 (T52) on RRM1, the catalytic subunit of ribonucleotide reductase (RNR). Consequently, this leads to RNR activation and increased production of dNTPs for efficient repairment of TMZ-induced DNA damage. Impairment in HPRT1-mediated AICAR production through genetic interruption or administration of 6-mercaptopurine (6-MP), a clinically approved inhibitor targeting HPRT1, impedes TMZ-induced AMPK activation and sensitizes brain tumor cells to TMZ treatment in mice. Furthermore, elevated levels of HPRT1 expression are positively associated with poor prognosis among GBM patients undergoing TMZ treatment. These findings unveil a pivotal dual role played by TMZ in GBM therapy that ultimately contributes to chemoresistance.
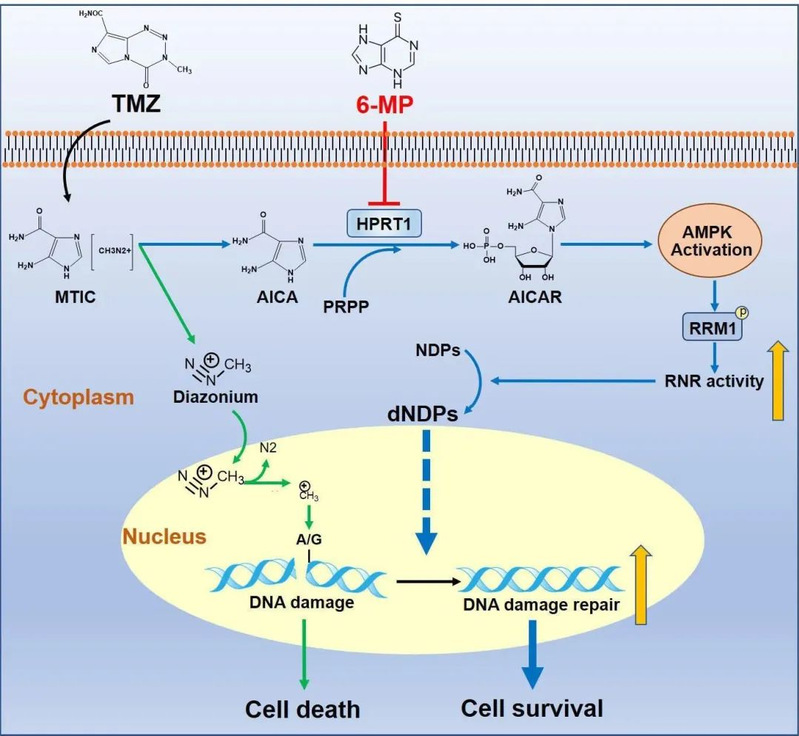
This study challenges previous perceptions of TMZ and uncovers its pivotal dual functionality in GBM therapy. Furthermore, this study underscores the synergistic impact of combining 6-MP and TMZ treatment on inhibiting brain tumor growth.
Prof. Xu Qian from the School of Public Health of Nanjing Medical University, Prof. Zhimin Lu from Institute of Translational Medicine of Zhejiang University and Prof. Yongping You from Department of Neurosurgery of The First Affiliated Hospital of Nanjing Medical University are the co-corresponding authors of this work. Yin Jianxing, Postdoctoral Fellow of Gusu College of Nanjing Medical University, Prof. Xiefeng Wang of Department of Neurosurgery of The First Affiliated Hospital of Nanjing Medical University, and Prof. Xin Ge of the School of Public Health of Nanjing Medical University, are the co-first authors of this work.
Link to original article: https://doi.org/10.1038/s41467-023-41663-2

